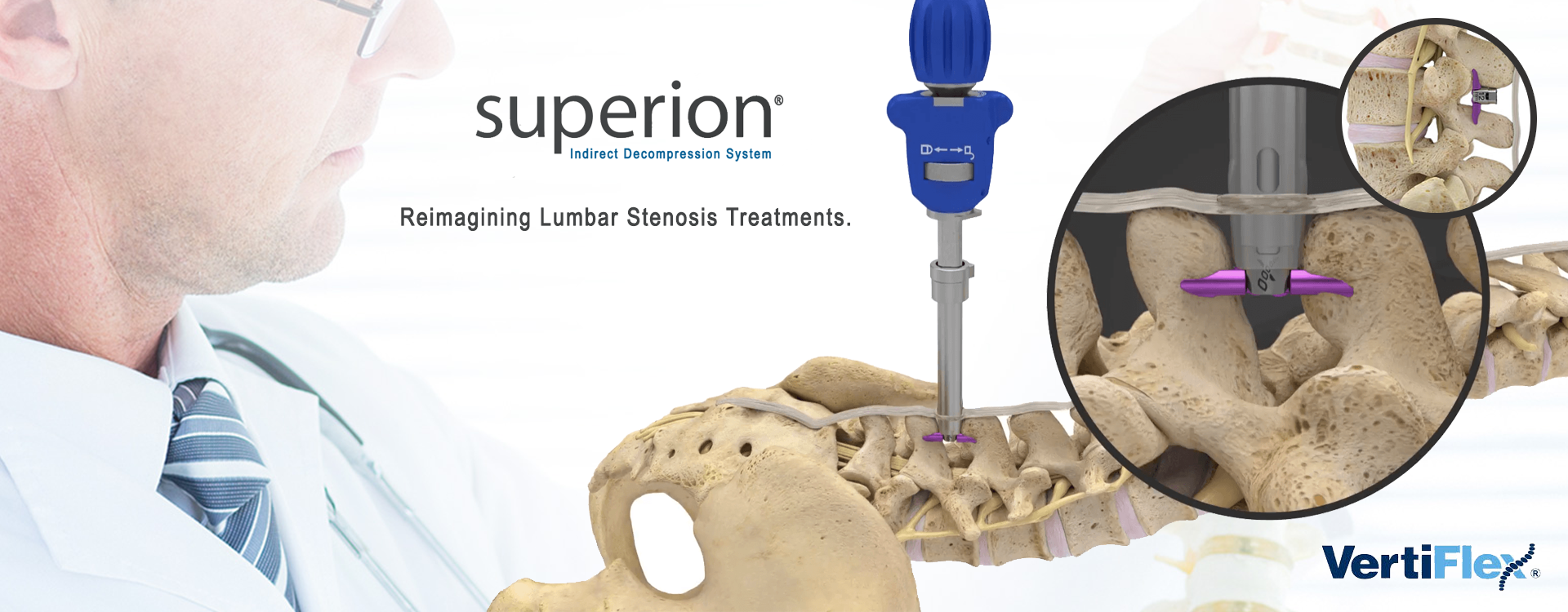
Superion Indirect Decompression System
Superion indirect decompression system. A SUPERION INDIRECT DECOMPRESSION SYSTEM COST on AVERAGE 12100. The Food and Druf Administration FDA recently approved a new treatment option for moderate Lumbar Spinal Stenosis LSS. AccessGUDID - Superion Indirect Decompression System 00884662000536- Superion Indirect Decompression System 10mm Skip to Main Content US.
The results published in the Journal of Pain Research showed an 85 decrease in the proportion of patients who were using opioids five years after. Superion is safe and effective and is an excellent choice for patients who havent yet found relief but want to avoid surgery. Do you have any opinion concerning the Superion Indirect Decompression System IDS.
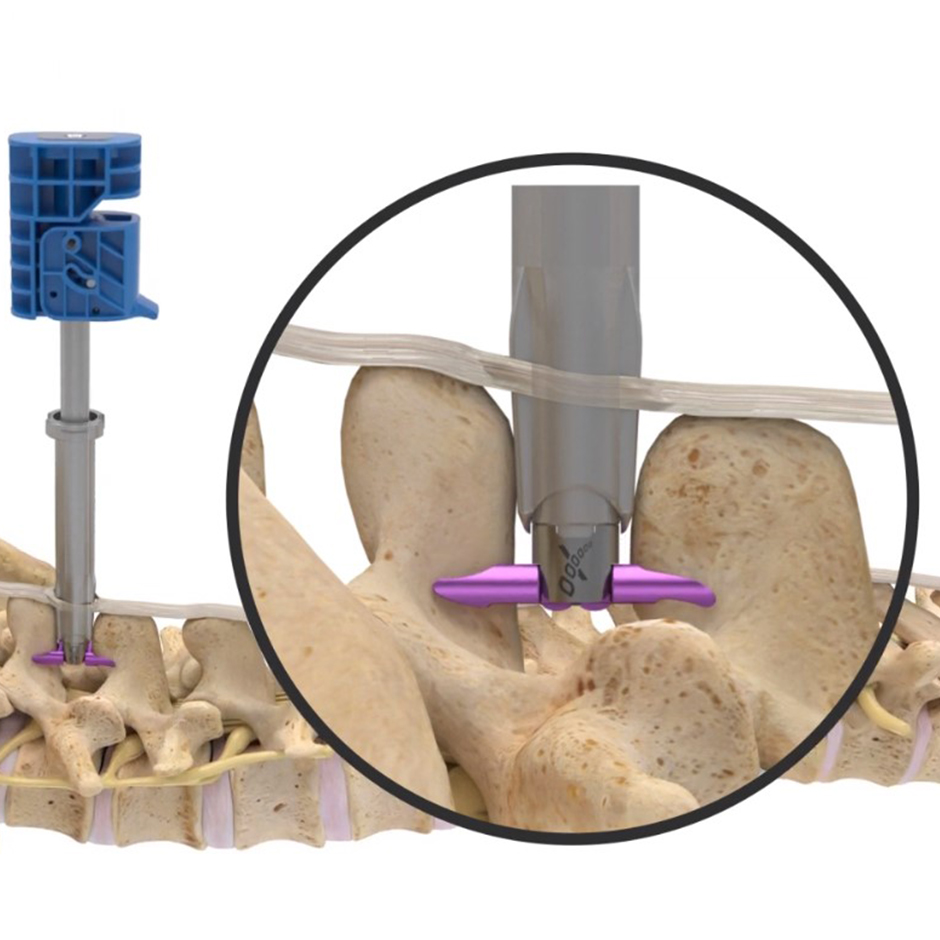
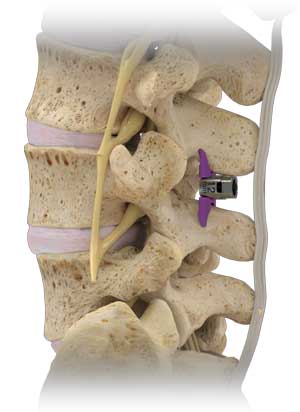
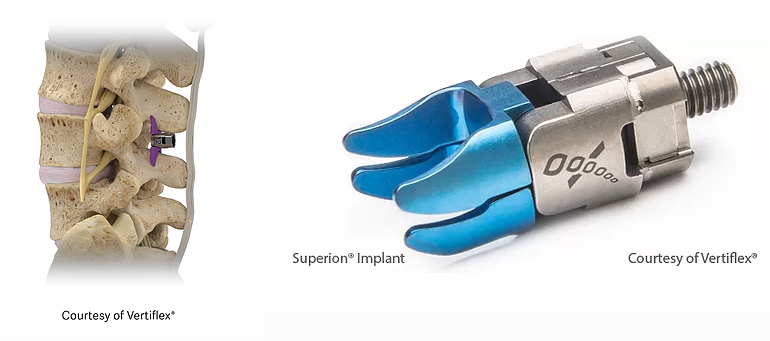
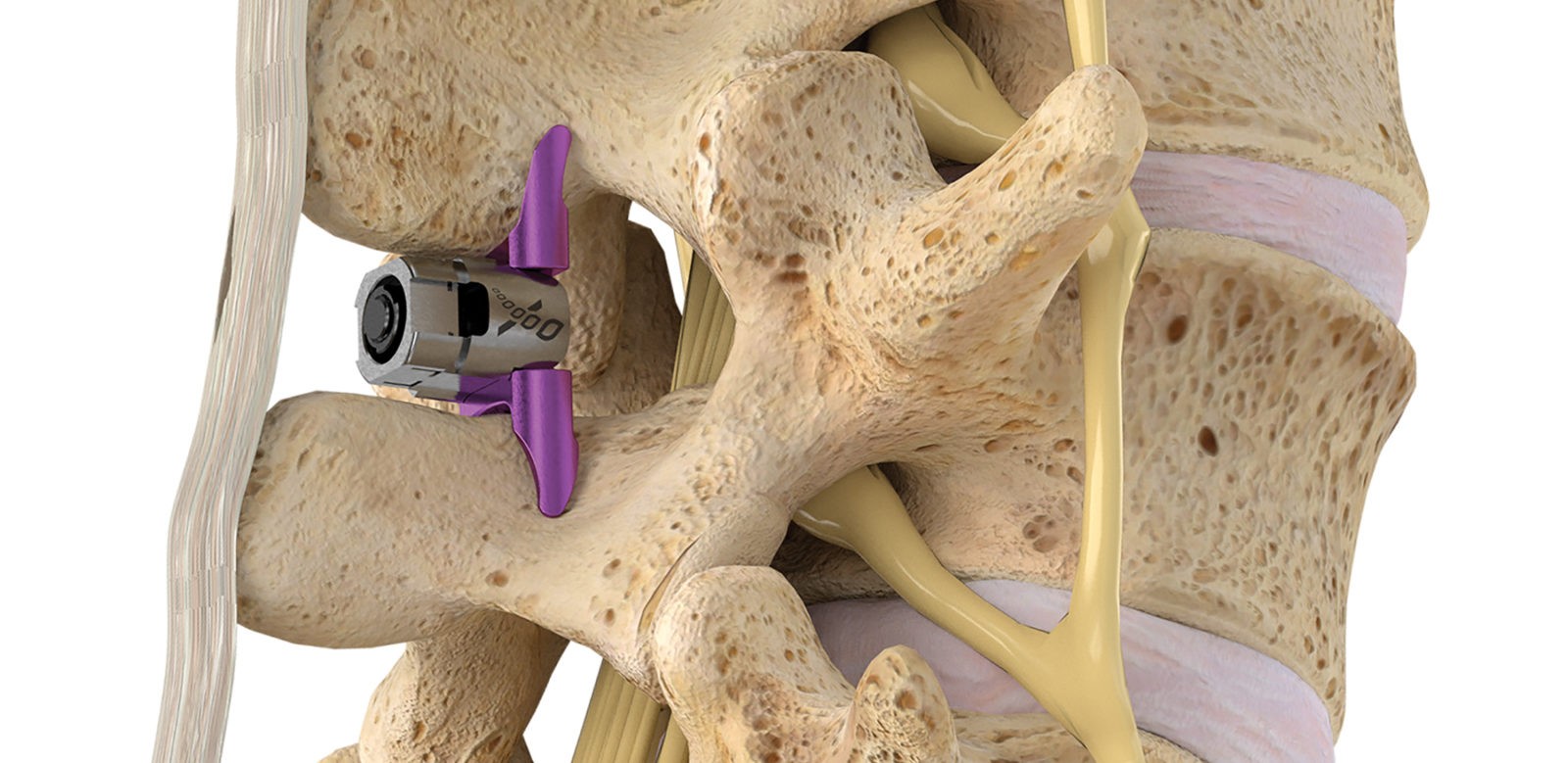
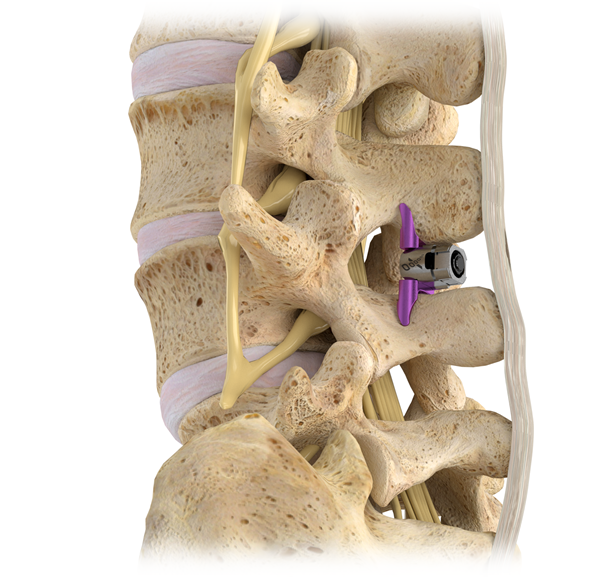
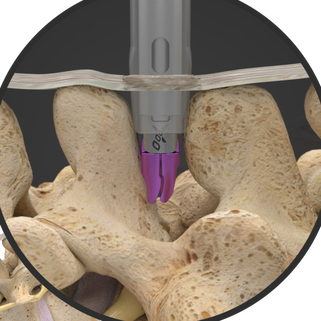
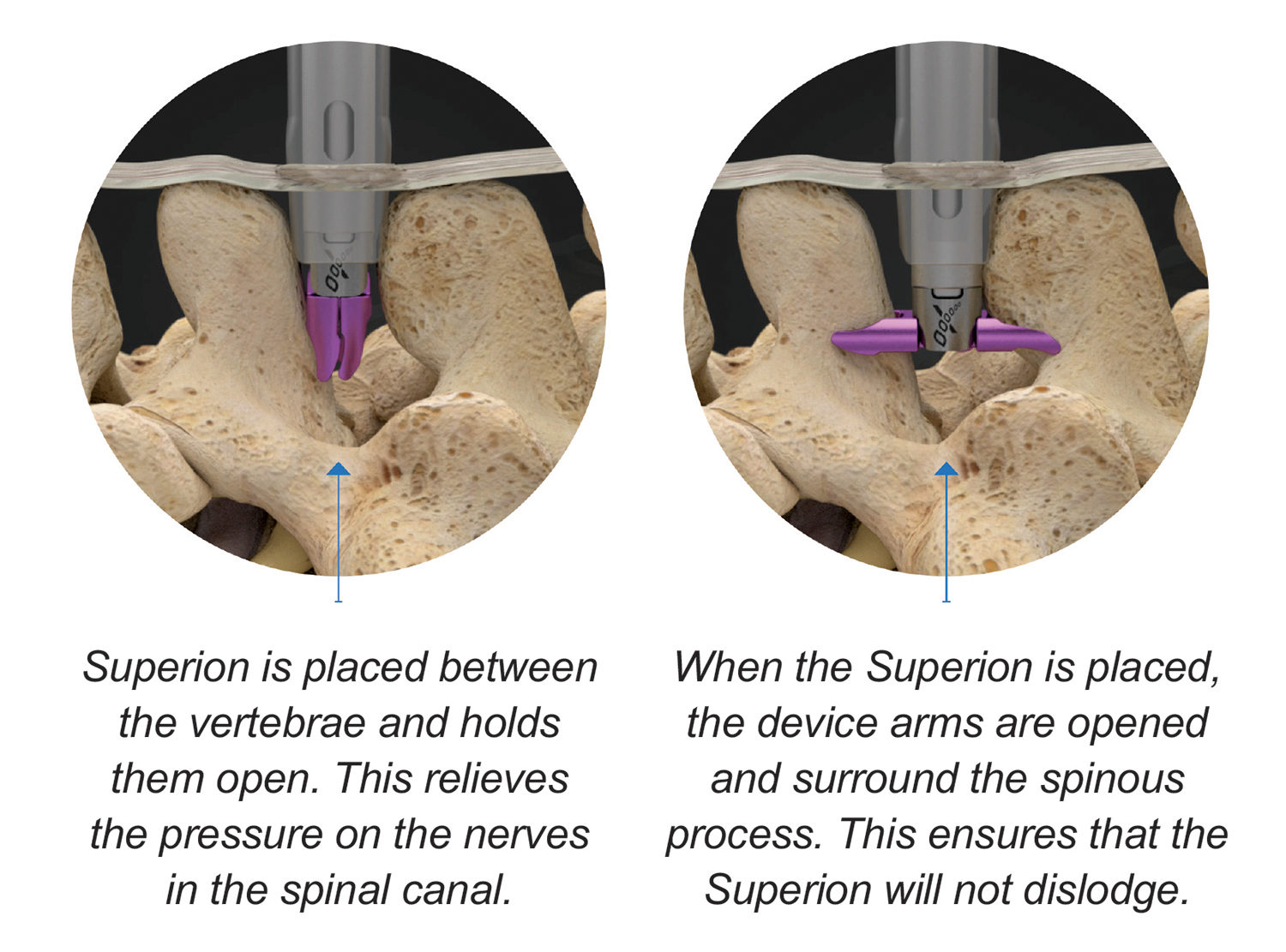
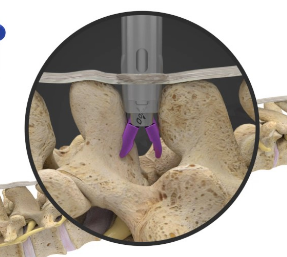
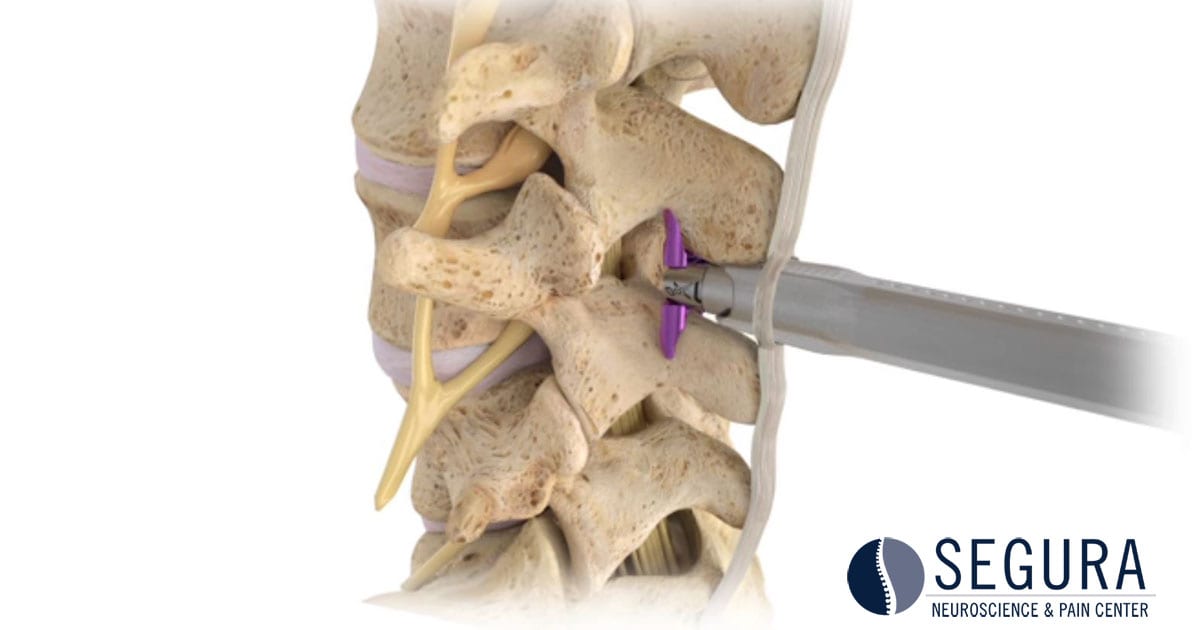
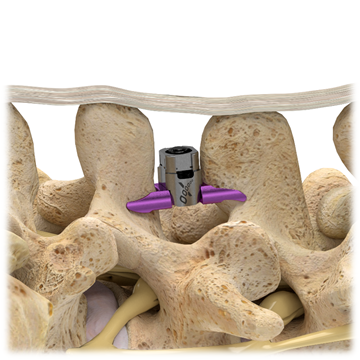
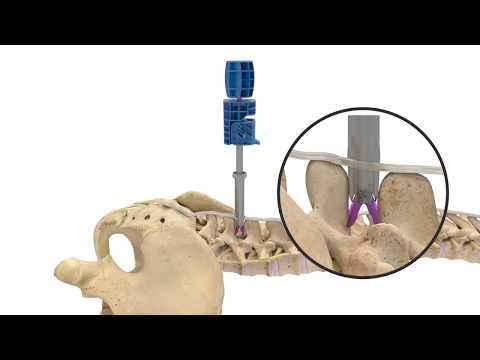
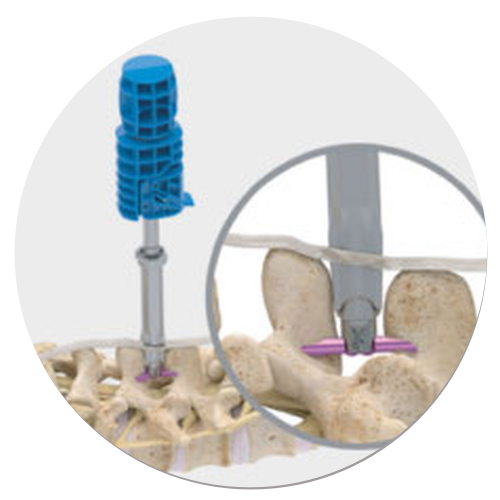
Superion is a minimally-invasive procedure. Non-clinical testing has demonstrated that the Vertiflex Procedure Superion Indirect Decompression System is MR Conditional. The Superion implant is a small titanium device that opens tight spaces in the spine caused by spinal stenosis.
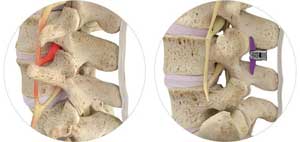
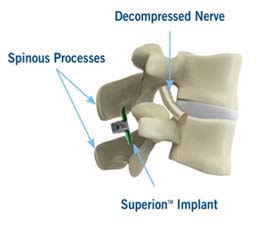
Superion Indirect Decompression System. A solution to LSS is Vertiflexs Superion Indirect Decompression System. When expanded the device relieves the compression on the spine experienced by patients with.
Static magnetic field of 15-Tesla 15 T or 30-Tesla 30 T. SUPERION INDIRECT DECOMPRESSION SYSTEM. Spatial gradient field of up to.
Vertiflex has announced additional results from a randomised controlled trial of its Superion Indirect Decompression System in patients with lumbar spinal stenosis LSS. It seems like it could be a good answer for my lumbar problems already have had two decompression surgeries at L45 L34 no hardware but I. SUPERION INDIRECT DECOMPRESSION SYSTEM - YouTube.
Superion Indirect Decompression System. The Vertiflex Procedure Superion Indirect Decompression System is redefining the treatment of LSS for patients.
The Food and Druf Administration FDA recently approved a new treatment option for moderate Lumbar Spinal Stenosis LSS.
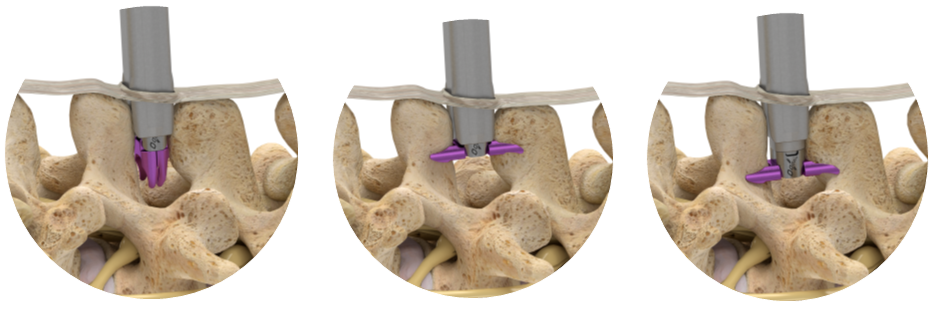
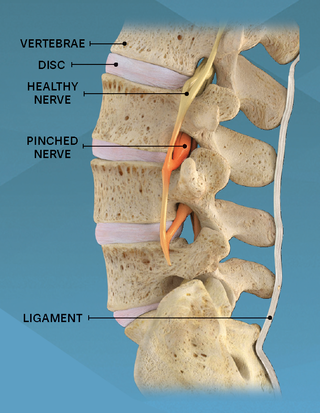
During this procedure your doctor will implant the superion device a small expandable spacer through a small incision in the lower back into the affected area of the spinal canal. Superion is a titanium implant designed to fit between vertebrae of the lumbar spine. The Superion Indirect Decompression System IDS is contraindicated for patients who. It can be scanned safely under the following conditions. When expanded the device relieves the compression on the spine experienced by patients with. Superion is designed to achieve indirect spinal decompression for patients suffering from neurogenic intermittent claudication pain or weakness in the leg caused by a problem at the nerve due to moderate lumbar spinal stenosis. Superion is a minimally-invasive procedure. Until the invention of the Superion Indirect Decompression System Superion the more invasive open surgical decompression surgery was often recommended for relief which could require hospitalization and posed a greater risk of infection and a longer road to recovery. SUPERION INDIRECT DECOMPRESSION SYSTEM - YouTube.
Non-clinical testing has demonstrated that the Vertiflex Procedure Superion Indirect Decompression System is MR Conditional. Indirect decompression is a device utilized to relieve pain by acting as an extension blocker in the spine. Spatial gradient field of up to. Superion represents a new minimally invasive approach to treating lumbar stenosis that fills a gap in the continuum between conservative care and. The Superion implant is a small titanium device that opens tight spaces in the spine caused by spinal stenosis. The Vertiflex Procedure is redefining the treatment of LSS for patients. Superion is a minimally-invasive procedure.






















Post a Comment for "Superion Indirect Decompression System"